Taipei, Taiwan, July 9, 2024 – Taiwan's dual legislation on regenerative medicine has cleared its third reading, marking significant progress in the fields of cell and gene therapy. With years of dedication in the field of cell therapy, MYCENAX has developed a comprehensive service for tailor-made process development and manufacturing. Leveraging advanced 3D bioreactor, they aim to accelerate the market entry of new therapies in the regenerative medicine industry, supporting urgent treatment needs for cancer and various diseases.
Technological Innovation and Process Optimization
MYCENAX continues to enhance its services and core technological platforms in the field of cell therapy products, addressing future development trends and overcoming technical challenges. They have established advanced 3D bioreactor applicable to multiple cell types, enabling industrial-scale production feasibility for stem cells, exosomes, and gene-edited immune cells like CAR-T. This addresses longstanding challenges in large-scale cell therapy production, achieving goals such as shortened production cycles, process automation, consistent product quality, and significant cost reductions.
Furthermore, MYCENAX is focusing on developing GMP-compliant downstream purification processes to enhance the yield and purity of exosomes, in response to the rising demand in this area.
Leading GMP Facility
As part of its commitment to quality, MYCENAX is nearing completion of a GMP facility for cell therapy at the Zhubei Biomedical Park in Taiwan. Equipped with world-leading isolators and automated bioreactors, this facility aims to deliver cell therapy drugs of the highest standard.
Streamlined Process from Development to Manufacturing
MYCENAX ensures seamless process development from research laboratories to GMP manufacturing facilities, reducing development and production times while maintaining consistently high quality.
About MYCENAX
MYCENAX is deeply rooted in the field of cell therapy, providing high-quality and efficient CDMO services throughout the stages of drug development, clinical trials, regulatory approval, and market launch.

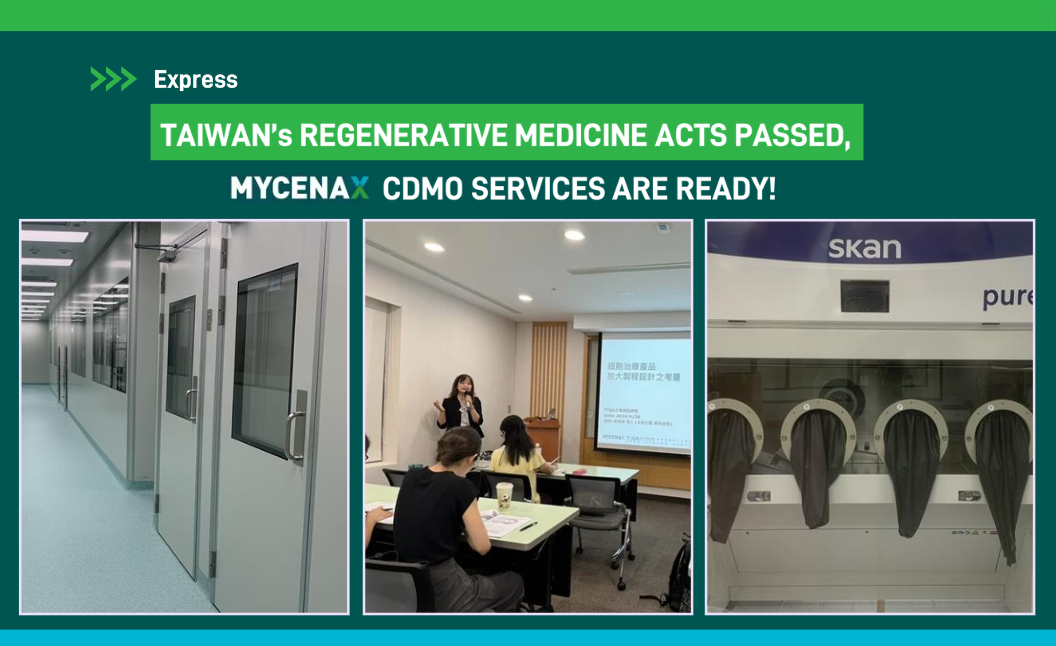
(Dr. Pei-Chi Tseng, Senior Manager of Cell Therapy Development at MYCENAX, delivered a lecture titled "Considerations on Scalable Process Design of CGT products " at the Taiwan Biomedical Quality Assurance Association on June 26, 2024.)

(The GMP cell therapy production facility at Zhubei Biomedical Park in Taiwan is nearing completion.)

Taipei, Taiwan, July 9, 2024 – Taiwan's dual legislation on regenerative medicine has cleared its third reading, marking significant progress in the fields of cell and gene therapy. With years of dedication in the field of cell therapy, MYCENAX has developed a comprehensive service for tailor-made process development and manufacturing. Leveraging advanced 3D bioreactor, they aim to accelerate the market entry of new therapies in the regenerative medicine industry, supporting urgent treatment needs for cancer and various diseases.
Technological Innovation and Process Optimization
MYCENAX continues to enhance its services and core technological platforms in the field of cell therapy products, addressing future development trends and overcoming technical challenges. They have established advanced 3D bioreactor applicable to multiple cell types, enabling industrial-scale production feasibility for stem cells, exosomes, and gene-edited immune cells like CAR-T. This addresses longstanding challenges in large-scale cell therapy production, achieving goals such as shortened production cycles, process automation, consistent product quality, and significant cost reductions.
Furthermore, MYCENAX is focusing on developing GMP-compliant downstream purification processes to enhance the yield and purity of exosomes, in response to the rising demand in this area.
Leading GMP Facility
As part of its commitment to quality, MYCENAX is nearing completion of a GMP facility for cell therapy at the Zhubei Biomedical Park in Taiwan. Equipped with world-leading isolators and automated bioreactors, this facility aims to deliver cell therapy drugs of the highest standard.
Streamlined Process from Development to Manufacturing
MYCENAX ensures seamless process development from research laboratories to GMP manufacturing facilities, reducing development and production times while maintaining consistently high quality.
About MYCENAX
MYCENAX is deeply rooted in the field of cell therapy, providing high-quality and efficient CDMO services throughout the stages of drug development, clinical trials, regulatory approval, and market launch.

(Dr. Pei-Chi Tseng, Senior Manager of Cell Therapy Development at MYCENAX, delivered a lecture titled "Considerations on Scalable Process Design of CGT products " at the Taiwan Biomedical Quality Assurance Association on June 26, 2024.)

(The GMP cell therapy production facility at Zhubei Biomedical Park in Taiwan is nearing completion.)

