Druggability
To offer comprehensive solutions to Mycenax’s customers, we provide one-stop shop services including cell line development, upstream cell culture process development, downstream purification process development, formulation development, analytical method development, process scale-up study, PIC/S GMP production and aseptic fill and finish.
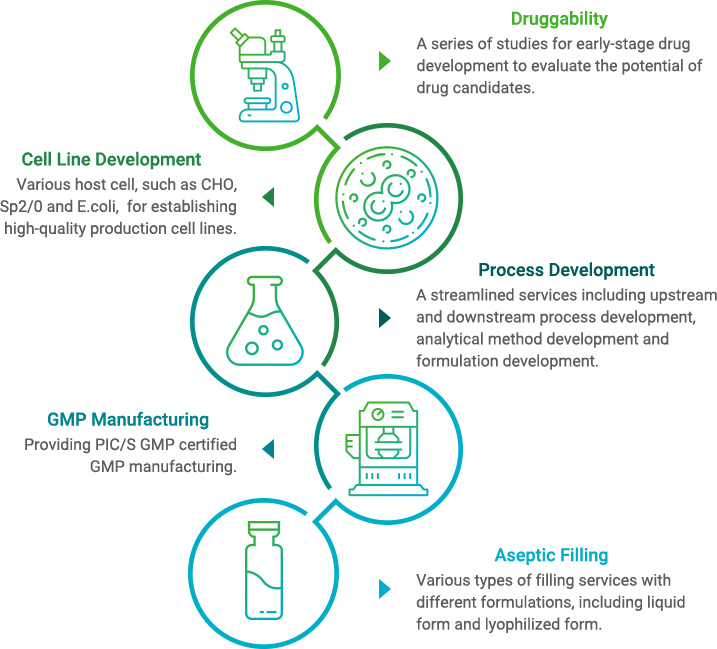
Protein drugs usually have individual differences in post-translational modification, solubility, aggregation, and stability. These differences are potential risks for drug development.
A good developability assessment can identify the differences mentioned above in advance and provide the basis for further process development. Through Mycenax’s extensive experiences, we tailor a complete set of developability studies for early-stage drug development to reduce the barriers for future products to enter large-scale production and PIC/S GMP production, and improve drug safety, effectiveness, and quality. Our developability studies can shorten the drug development timeline, cost, and failure rates.
|
 |
||||||||||||||||||||||||||
To offer comprehensive solutions to Mycenax’s customers, we provide one-stop shop services including cell line development, upstream cell culture process development, downstream purification process development, formulation development, analytical method development, process scale-up study, PIC/S GMP production and aseptic fill and finish.
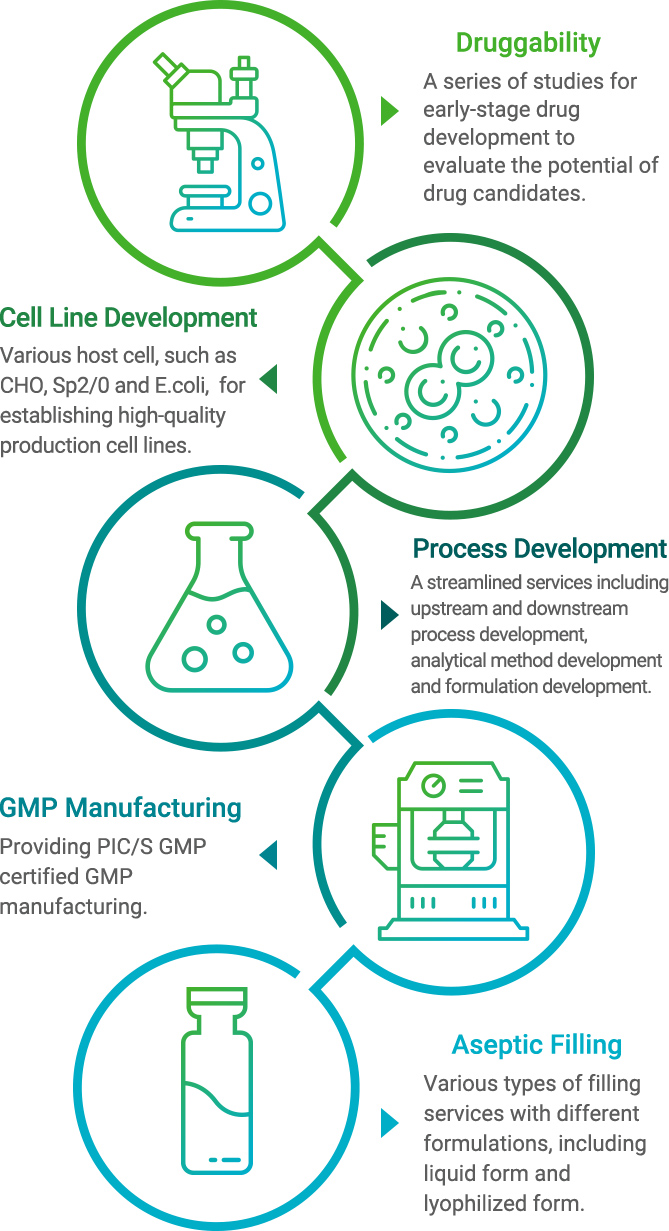
Protein drugs usually have individual differences in post-translational modification, solubility, aggregation, and stability. These differences are potential risks for drug development.
A good developability assessment can identify the differences mentioned above in advance and provide the basis for further process development. Through Mycenax’s extensive experiences, we tailor a complete set of developability studies for early-stage drug development to reduce the barriers for future products to enter large-scale production and PIC/S GMP production, and improve drug safety, effectiveness, and quality. Our developability studies can shorten the drug development timeline, cost, and failure rates.
|
||
|
◆ In silico analysis |
||
|
◆ Sequence and structural analysis |
||
|
◆ Heterogeneity |
||
|
◆ Product yield and productivity |
||
|
◆ Propensity of aggregation |
||
|
◆ Physical stability |
||
|
◆ Chemical stability |
||
|
◆ Post-translational modifications |
||
|
◆ Affinity analysis |
||
|
◆ Potency analysis |
||



