|
Mycenax’s state-of-the-art biomanufacturing site is PIC/S GMP certified. Our highly qualified and well-trained team routinely manufactures high-quality biopharmaceutical products by mammalian cell culture and microbial culture processes. We continuously invest in our technologies and infrastructure to ensure that we constantly meet your evolving needs of today and tomorrow.
|
|
|
|
The innovative design of our multi-product GMP facilities allows for capacity and technological flexibility while ensuring strict compliance with biopharmaceutical regulations required for GMP operations. Our dedicated and experienced technical teams are committed to delivering high-quality products rapidly and cost-effectively.
|
| |
 |
|
Mycenax conducts cell banking in compliance with GMP requirements. Our GMP-grade cell banks, such as master cell banks and working cell banks, meet global quality standards and are manufactured in accordance with ICH and global regulatory requirements. We also take over the complete biosafety and characterization of your cell banks in accordance with ICH and global regulatory requirements. For the establishment of cell banks, we can provide services as follow:
|
|
◆ Master cell bank (MCB) production
|
|
◆ Working cell bank (WCB) production
|
|
◆ Cell bank characterization
|
|
◆ Cell bank storage
|
|
|
 |
|
| |
 |
|
|
|
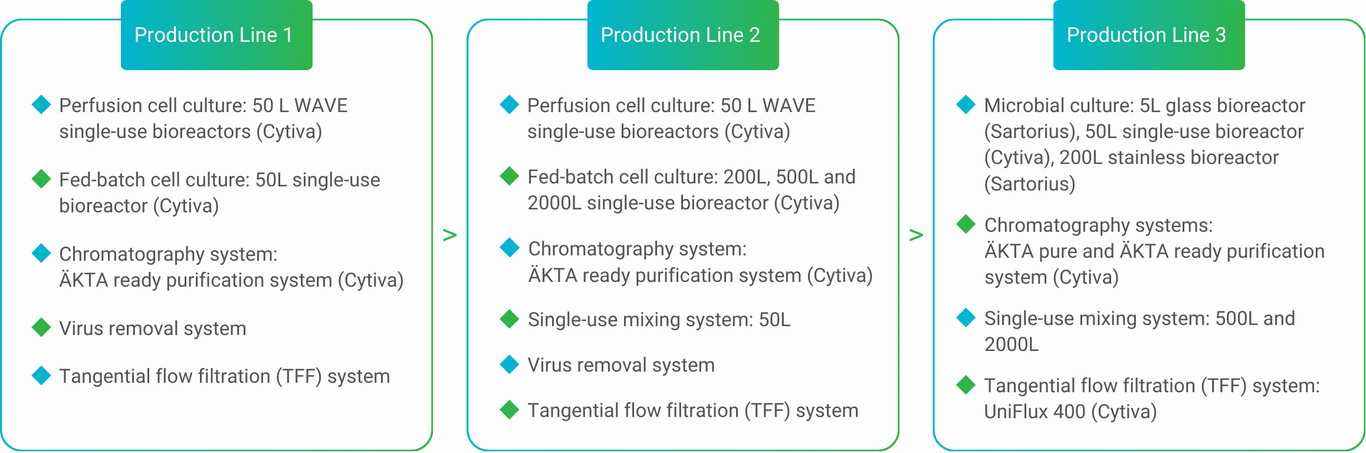
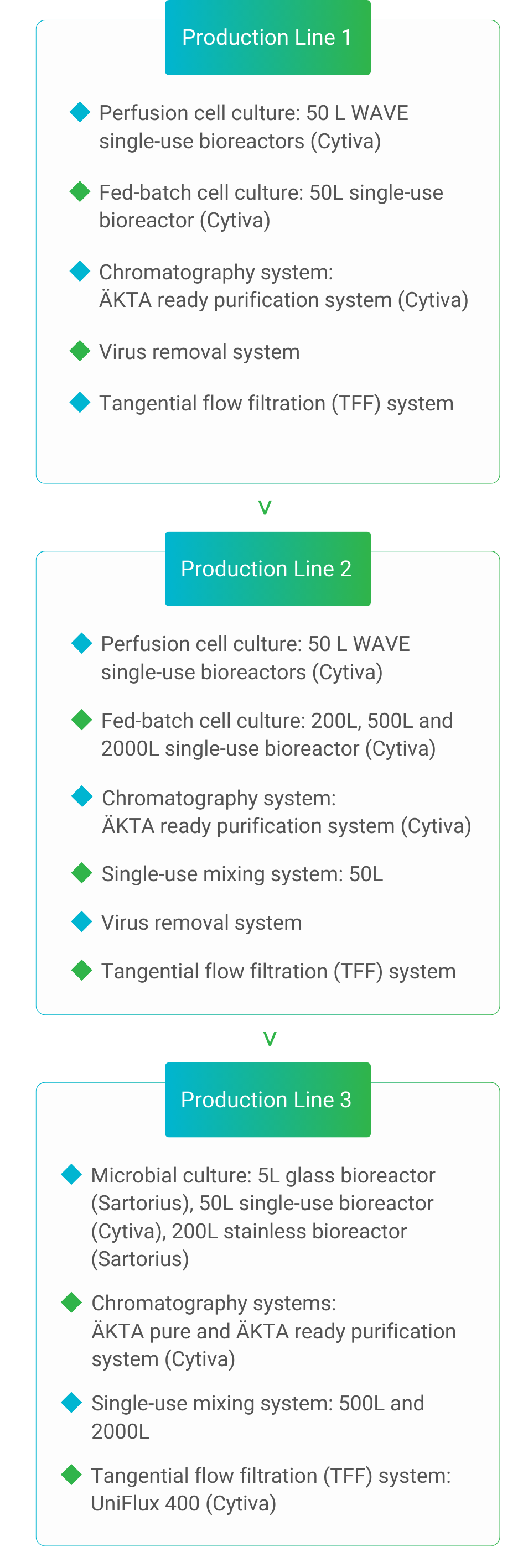
Mycenax manufactures high-quality drug substances by fed batch or continuous perfusion cell culture processes meeting GMP or non-GMP requirements for the respective development stage.
|
|
|
 |
|
| |
|

Mycenax offers an extensive range of aseptic filling services, catering to both liquid and lyophilized formulations designed for 2R, 4R, and 10R vials, as well as liquid prefilled syringes (PFS) and cartridges. Our two filling lines are designed to handle these diverse forms, ensuring both flexibility and efficiency in the production of drug products across all clinical phases and commercial purposes. By employing single-use technology across our filling lines, Mycenax facilitates rapid process changeovers and robust cross-contamination prevention measures. At Mycenax, our state-of-the-art facilities and advanced technologies are dedicated to delivering efficient and high-quality aseptic filling services.
|
|
|
 |
|
Filling Line 1, located in GMP Facility 1, features a multifunction filler, a 0.9 m2 Freeze Dryer capable of processing 900 to 1200 pieces per hour, and an Open Restricted Access Barrier System (OPEN RABS). This system creates a physical barrier between personnel and the production area, maintaining the Class A clean area in a sterile environment and minimizing the risk of product contamination.
Mycenax offers various types of filling services for customers with different formulations, including vials, pre-filled syringes and cartridges in liquid form. In addition, we can produce drug products in lyophilized form.
|
|
| |
 |
|
Filling Line 2, located in GMP Facility 2, is outfitted with a state-of-the-art multifunction filler (Aseptic Filling and Closing Machines OPTIMA SV125) enclosed within an Isolator, along with a 10 m2 Freeze Dryer. The OPTIMA SV125 features an automatic loading/unloading system, delivering impressive processing speeds of 6,000 vials per hour and 12,000 pieces per hour for prefilled syringes.
|
|
Guidance:
|
|
I. ICH Q7: Good Manufacturing Practice Guide for Active Pharmaceutical Ingredients
|
|