Exosome Manufacturing Platform
 |
|||||
| What are exosomes? | |||||
| Exosomes are small extracellular vesicles (EV) that play a crucial role in cell-to-cell communication and hold great potential for early diagnosis, prognosis, and targeted therapy. Their unique properties make them a promising next-generation biologic. | |||||
| Bridging Exosomes Innovation to Clinic with the ExoMX™ Platform | |||||
| To overcome the manufacturing challenges of MSC-EV products in clinical translation, we have developed ExoMX, a state-of-the-art, scalable, and GMP-compliant platform. This platform integrates a 3D microcarrier-based bioreactor process with TFF and chromatography-based purification to ensure high-quality EV production. | |||||
 |
|||||
 |
|||||
 |
|||||
 |
|||||
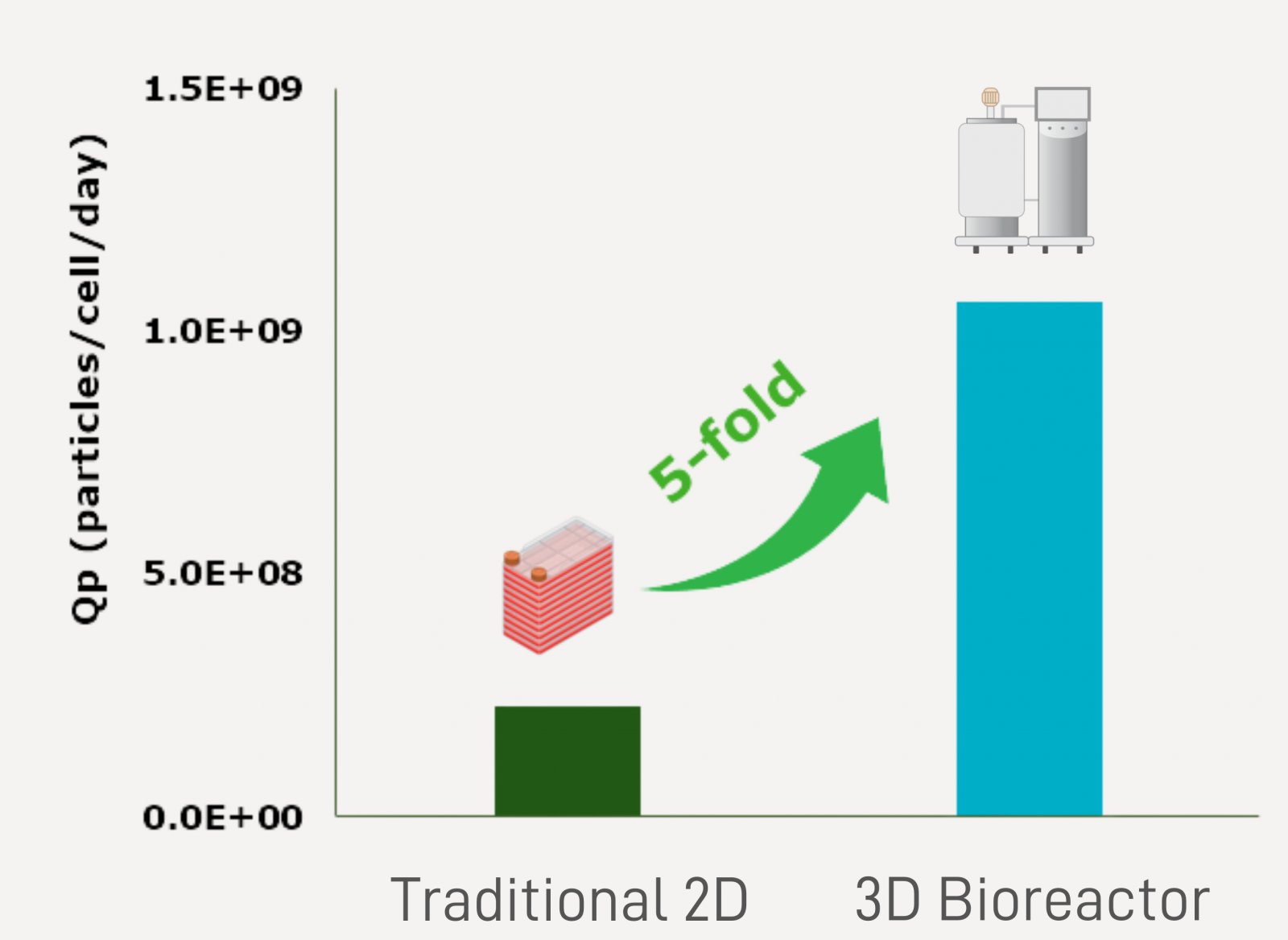
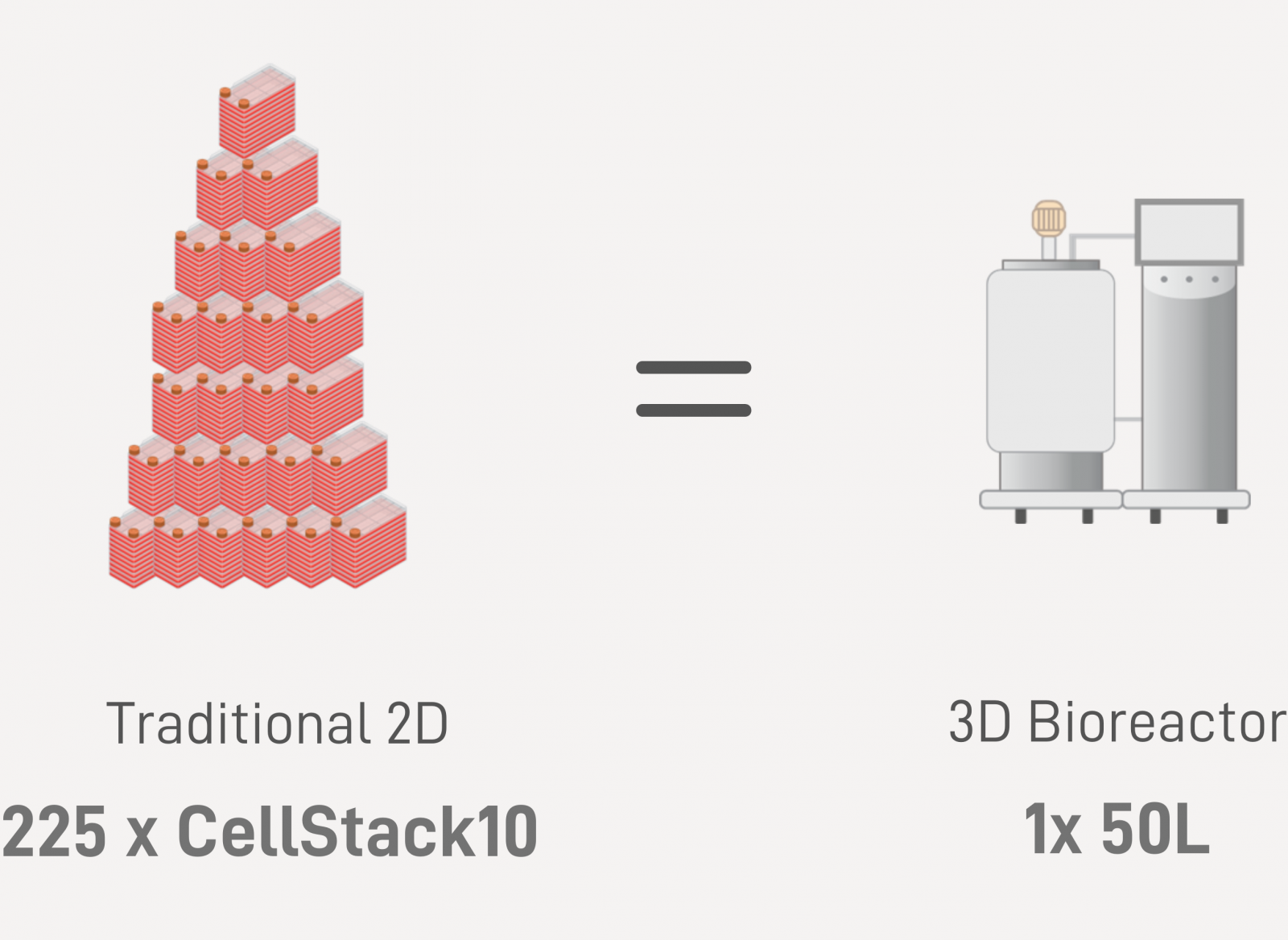
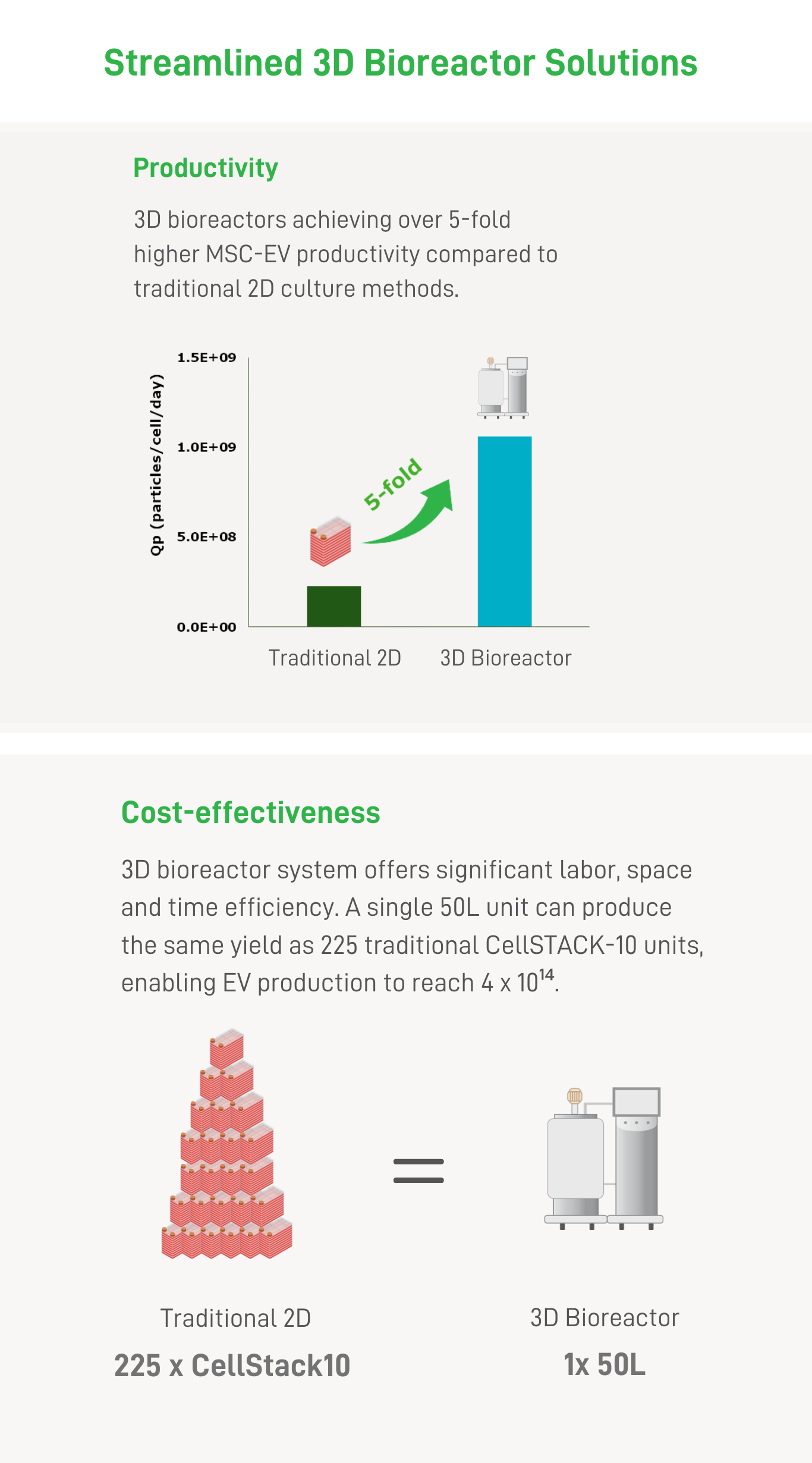
| Industrialized EV Production: Accelerate Market Entry by Advanced 3D Bioreactor Technology | |||||
 |
|||||
|
With 3D-boosting technology and streamlined bioreactor manufacturing capabilities ranging from 0.25L to 50L scale, we support product development path into clinical use.
|
|||||
 |
|||||
| Flexible Solutions for every stage | |||||
| We offer phase-appropriate manufacturing, versatile purification strategies, and tailored analytical services. Built on GMP principles, our step-by-step approach minimizes risk and accelerates your path to market. | |||||
 |
|||||
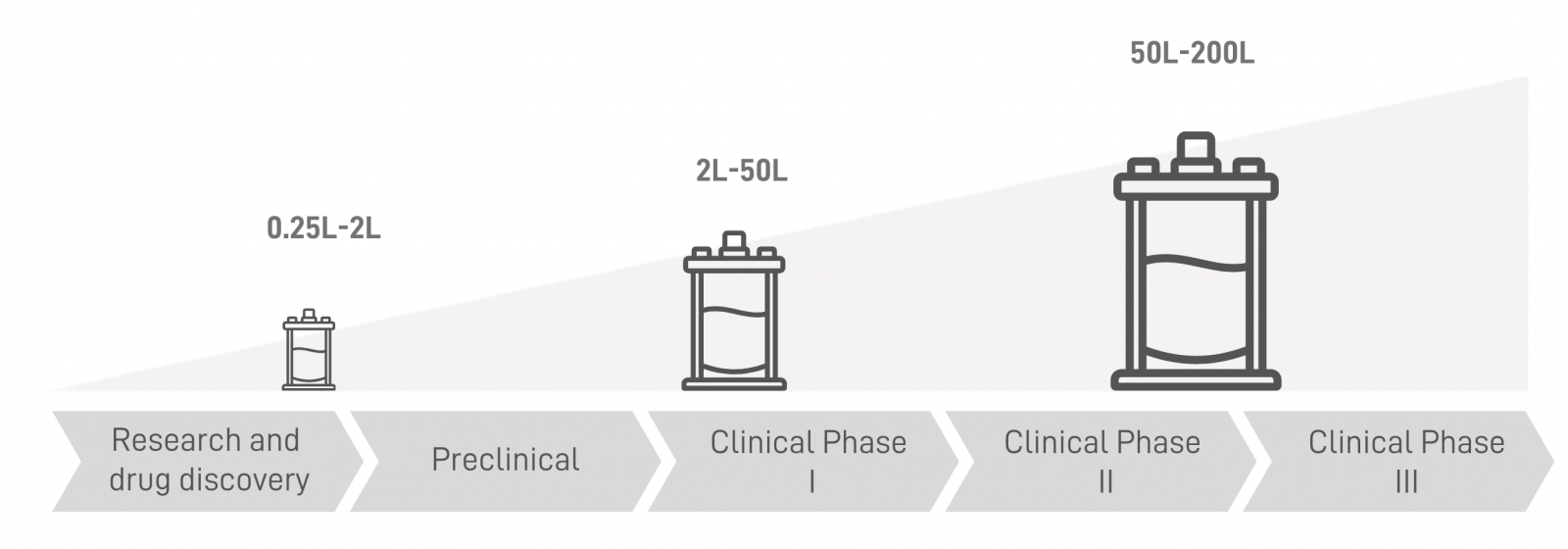
| Phase-appropriate Manufacturing Scales | |||||
|
Enabling seamless advancement from early R&D to clinical transition. |
|||||
 |
|||||
 |
|||||
|
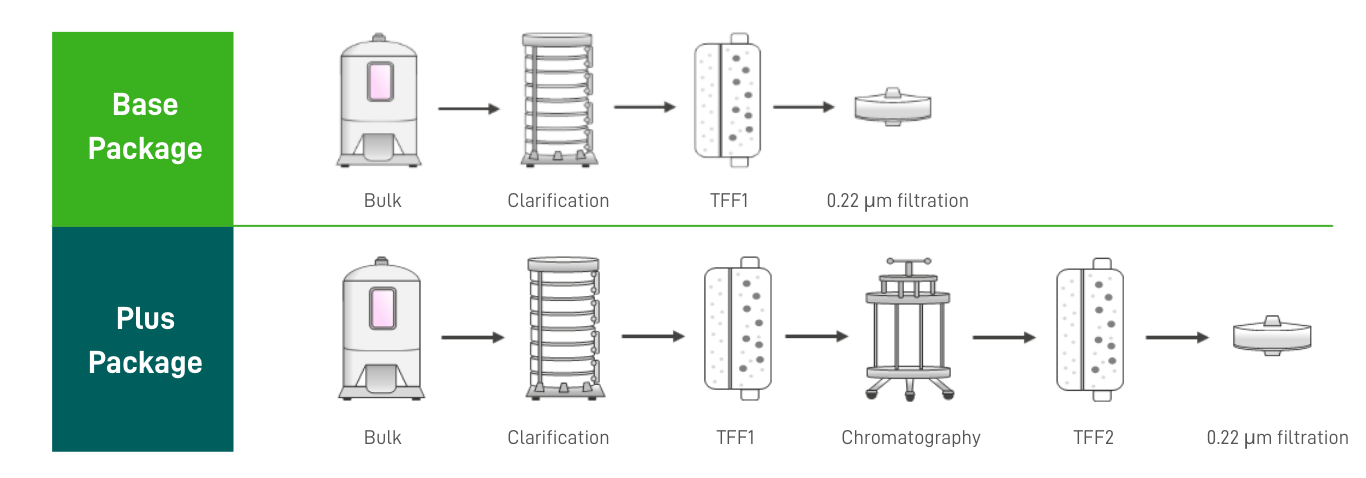
Versatile Exosome Purification Strategies |
|||||
|
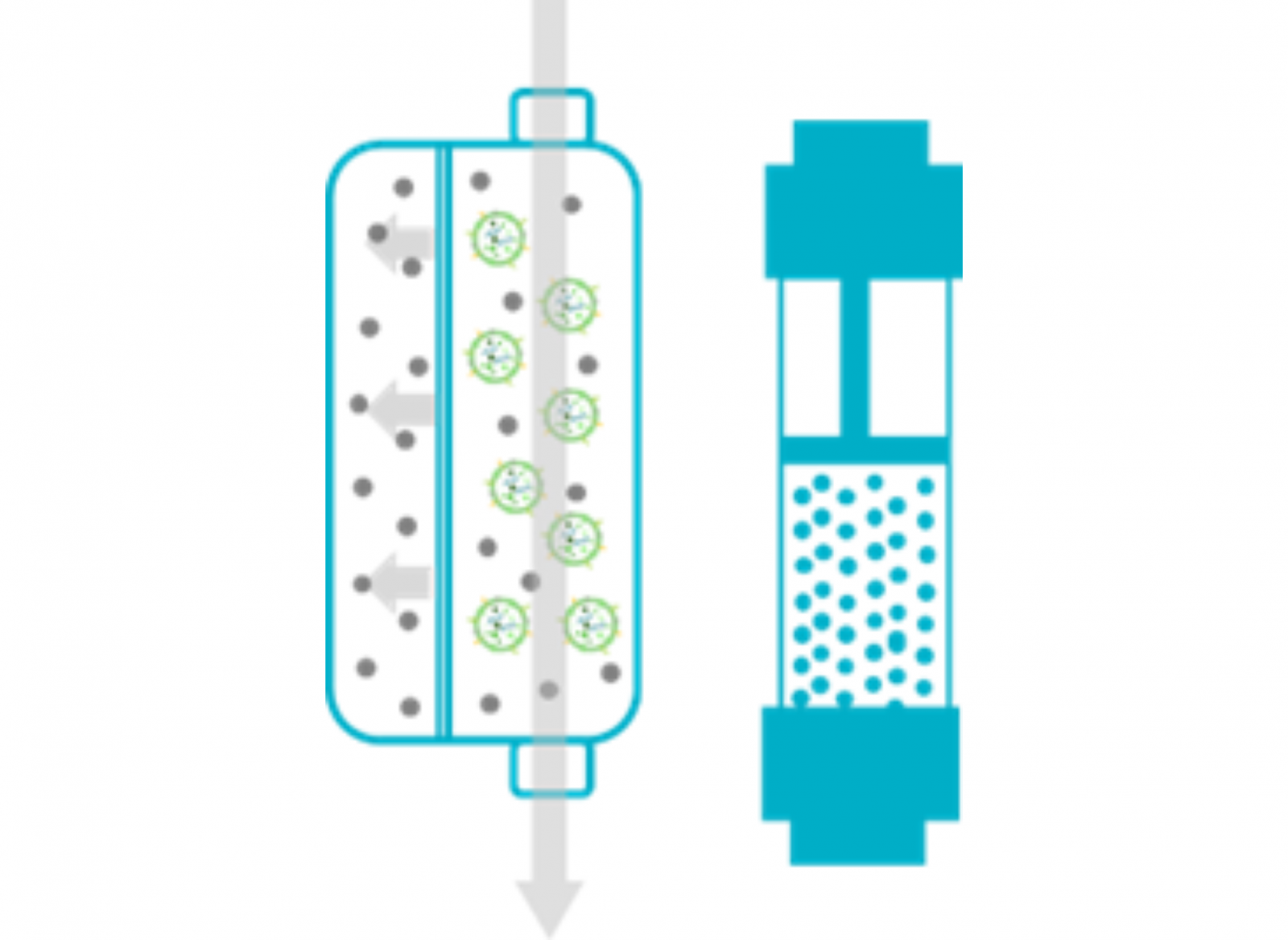
Downstream processing is critical for final yield, batch consistency, and product potency. Our experts develop scalable, customized workflows from medium harvest, exosome purification and vialing. With advanced equipment and expertise, we tailor every step to meet your specific needs. |
|||||
 |
|||||
 |
|||||
 |
|||||
|
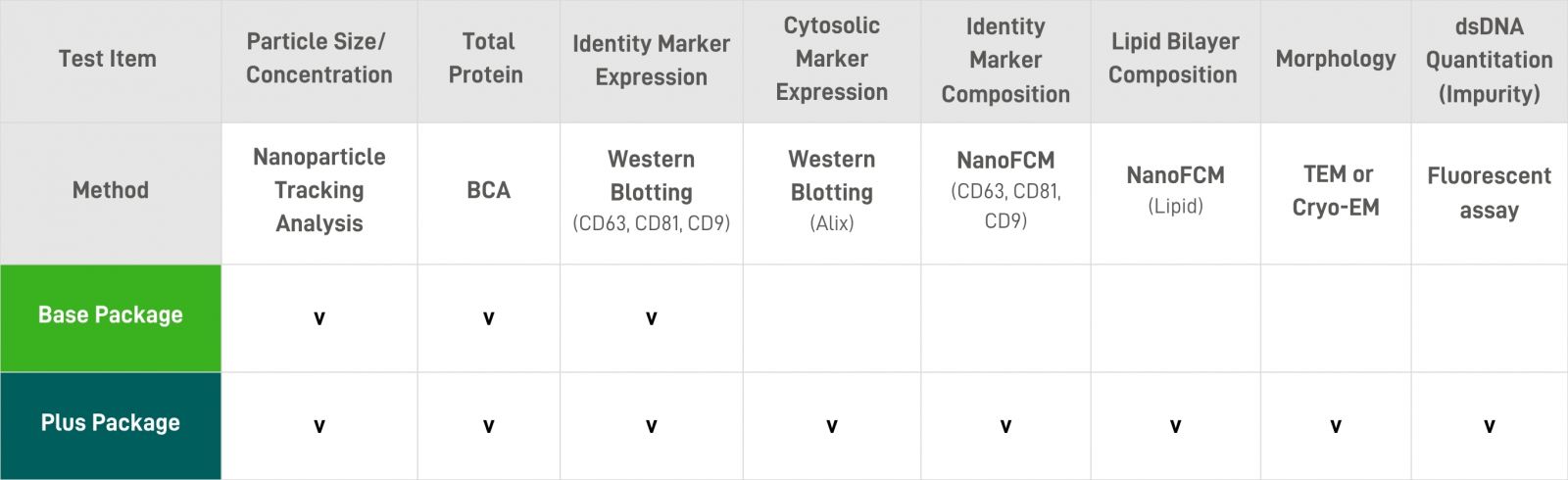
Analytical Services |
|||||
|
We offer comprehensive EV Characterization Packages, including Base and Plus options. All assays can be chosen individually as you need. |
|||||
 |
|||||
|
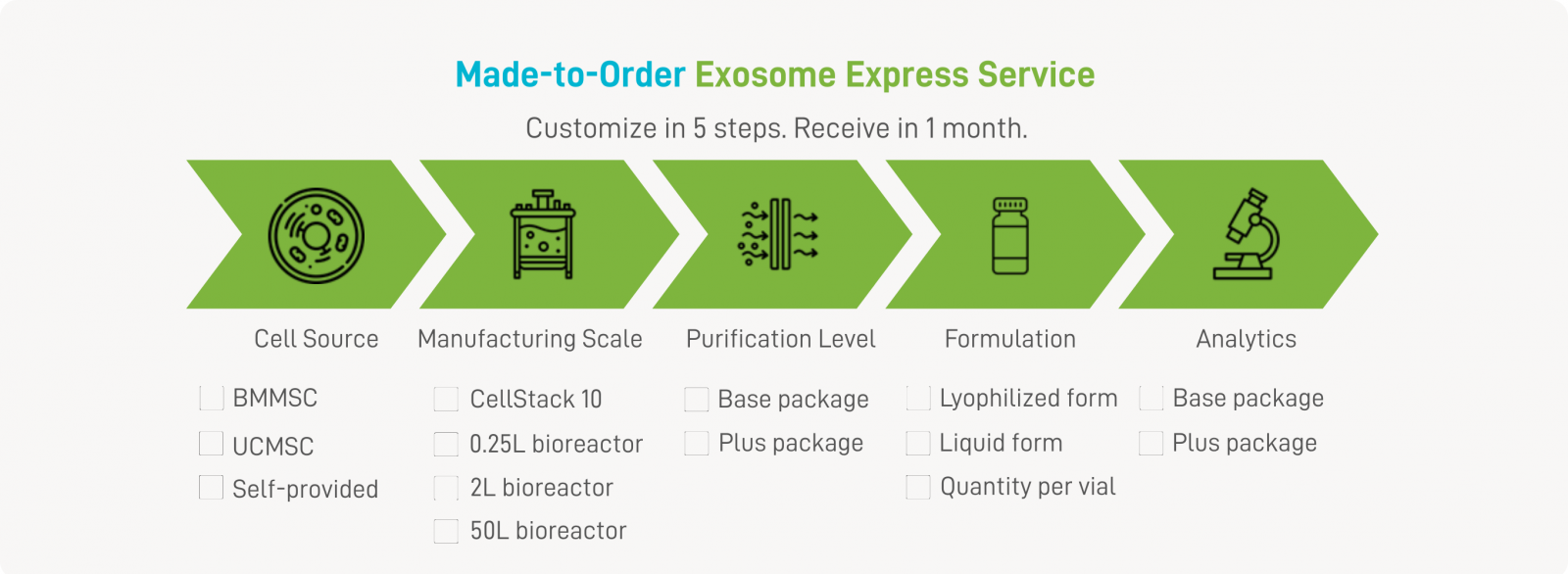
From Click to Delivery — Your Exosomes, in 30 Days. |
|||||
|
Accelerate your research with Mycenax's Exosome Express Service — a fast, flexible, and high-quality solution for obtaining human MSC-derived exosomes. Powered by our ExoMX™ platform, pre-established human MSC research cell banks, and a xeno-free, chemically defined process, we offer both customized and ready-to-ship options to meet your specific needs. |
|||||
 |
|||||
 |
|||||
|
|
|||||
 |
|||||